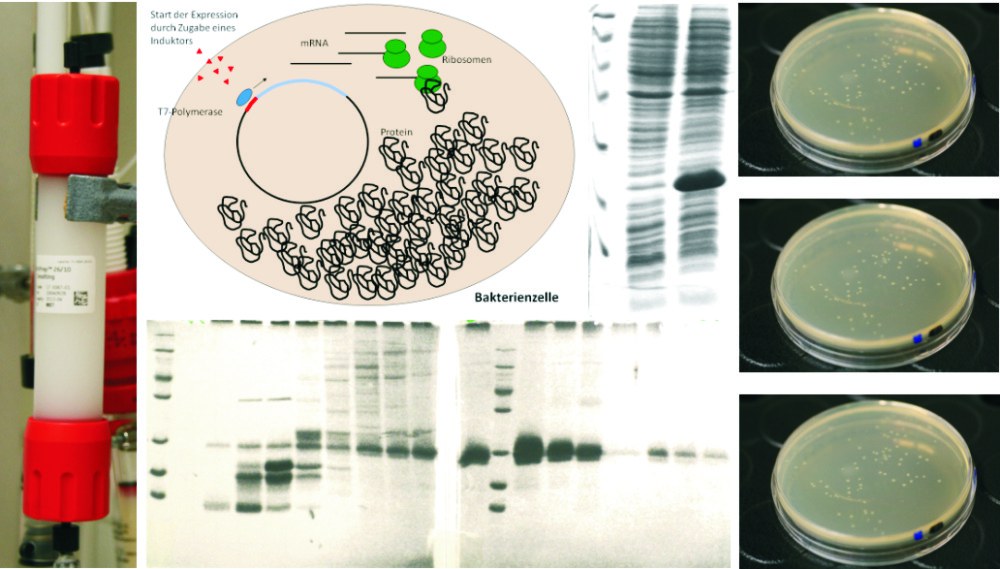
Expression and purification of recombinant proteins
Various biophysical methods require large amounts of recombinantly expressed and purified proteins.
Additionally, solving the three-dimensional structure of proteins by NMR requires the production of isotope-labeled proteins. Therefore, we use efficient recombinant expression systems, which are optimized depending on the target protein. Besides E.coli, the expression systems also include yeasts, human or animal cell lines and insect cells. The expressed proteins are subsequently purified using biochemical methods such as affinity, ion exchange, size exclusion or hydrophobic interaction chromatography, or are subjected to tag-based purification procedures.
Last Modified: 25.05.2022
