Autophagy
Autophagy is the highly conserved process of degradation of cellular components in eukaryotes. Autophagy plays an important role in the basic turnover of intracellular proteins and organelles, as well as in the production of amino acids under starvation conditions. In this context, the contribution to protein degradation by autophagy is similar to that of the ubiquitin-proteasome system. Furthermore, autophagy plays a role in antigen presentation or degradation of invasive bacteria. Disturbances in the regulation of autophagy can lead to neurodegenerative diseases, but also to cancer. During autophagy, the proteins and organelles to be degraded are enclosed by a so-called isolation membrane. The initially crescent-shaped double membrane grows until its ends fuse and form the autophagosome. Several proteins are required for this process; among them proteins of the GABARAP family.
GABA receptor-associated protein (GABARAP) belongs to a protein family involved in intracellular transport processes as well as autophagy. It includes several human members, such as GABARAP and MAP LC3 (microtubule-associated protein 1 light chain 3), but also the yeast protein Atg8 (autophagy-related protein 8).
ABARAP was originally discovered as a ligand of the receptor for γ-aminobutyric acid (GABA) type A (GABAA receptor). However, it is now clear that GABARAP plays a much more important and general function in general vesicle transport processes, most notably during autophagy.

We were the first to determine both the solution structure of the human protein GABARAP, and the solution structure of the yeast protein Atg8. They show the structure known for members of the ubiquitin superfamily, which is characterized by the so-called β-grasp fold. It consists of a central four-stranded β-grasp with two α-helices on its concave side. A distinctive feature of the GABARAP family is an N-terminal extension with two additional α-helices located on the convex side of the β-sheet. This N-terminal domain exhibits conformational flexibility at Atg8 on the micro- to millisecond time scale. In contrast, the N-terminus of GABARAP exhibits a direct interaction with the C-terminus.
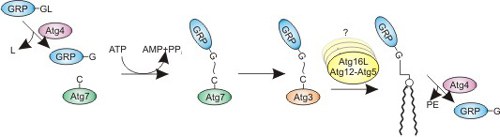
ABARAP and GABARAP-homologous proteins can be reversibly lipidated and anchored to membranes in the cell. This and the conformational flexibility in the N-terminal region can potentially promote oligomerization of lipidated protein, facilitating, for example, autophagosome formation.
